The World Health Organization states the leading cause of death in 2050 to be from resistant bacterial infections. The goal of our research is to bring new nanomedicine treatments to patients with difficult-to-treat bacterial infections. These fall under two main categories: Multidrug-resistant bacteria and chronic bacterial biofilm diseases. Our group aims to solve the unmet medical needs to treat chronic biofilm infections and infections caused by resistant bacteria through the engineering of novel nanoparticles (e.g., inorganic and polymeric nanostructures) as well as the development of novel treatment modalities. We approach this work from an observation-initiated and hypothesis-driven manner.
Our research program bridges bio-sciences, material sciences, chemistry, engineering, and pharmaceutics with clinical applications. Our Research team lead by Dr. Bekale consists of a multidisciplinary team of students, clinician-scientists, and basic research scientists from various disciplines and backgrounds. Researchers in the lab acquire a broad range of skills, including nanomaterial design, microbiology, and cell biology laboratory techniques (i.e., cell culturing, cytotoxicity testing, biofilm testing, antibiotic resistance testing, cytotoxicity testing, minimum inhibitory and minimal bactericidal concentration testing), and animal handling.
Discovering Novel Bacteria-Nanoparticle Interactions
Our hope is that through a fundamental understanding of bacteria-nanoparticle interactions, will lead to the design of innovative therapeutic strategies
Engineering nanoparticles to treat infection caused by multidrug-resistant bacteria. We are exploiting bacteria-nanoparticle interactions to develop nanoparticle-specific rules (i.e., structure-property-function relationships) that drive bacterial cell death. Understanding these rules is essential for the rational design of effective nanoparticles to combat bacteria resistance.
Cao, Zhixin, Jing Chen, Jessica Tran, Xiaohua Chen, Brian Bacacao, Laurent A. Bekale, and Peter L. Santa Maria. “Antimicrobial Gold Nanoclusters Eradicate Escherichia Coli Biofilms and Are Nontoxic by Oral Administration.” ACS Applied Bio Materials 3, no. 8 (August 17, 2020): 5275–86. https://doi.org/10.1021/acsabm.0c00641.
Engineering nanoparticles as novel antibiotics adjuvants. We are exploiting bacteria-nanoparticle interactions to affect the ability of bacteria to develop antibiotics cross-resistance. Preventing cross-resistance development that can offer a way to mitigate the gap between the need for new drugs and the diminishing supply antibiotics pipeline.
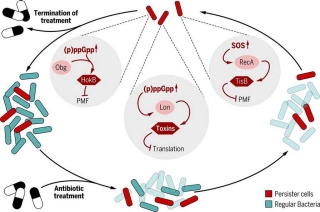 Our unique nanomedicine approach prevents antibiotic resistance from developing and enables the unique targeting or persistent and resistant bacteria.
Our unique nanomedicine approach prevents antibiotic resistance from developing and enables the unique targeting or persistent and resistant bacteria.

Principal Investigator

Lead Research Scientist
